|
Lecture " High pressure phase behavior of systems with hyperbranched polymers " Theo W. de Loos * , Christian S. Schacht, Thijs J.H. Vlught, Joachim Gross Delft University of Technology, Department Process&Energy/Engineering Thermodynamics, e-mail: t.w.deloos@tudelft.nl, tel: +31-(0)15-278-8478/6651 Abstract Hyperbranched polymers are a relatively new class of polymers that can be easily synthesized via one-step reactions and represent economically promising products for large-scale industrial applications. The properties of hyperbranched polymers can be adjusted by controlled functionalization of the end groups. They constitute selective solvents that can be tailored for a specific task. Seiler et al. [1] have shown the applicability of hyperbranched polymers as new selective solvents for extractive distillation and solvent extraction. Not much is known about the phase behavior of systems with hyperbranched polymers. In this work, a review of the research on this topic in the Engineering Thermodynamics group in Delft will be presented: 1) The phase behavior of the system carbon dioxide + boltorn H3200, propane + boltorn H3200 and butane + boltorn H3200 was investigated experimentally. In the system CO 2 + boltorn fluid phase equilibria were measured at pressures up to 400 MPa. The system probably belongs to type III of fluid phase behavior with a metastable liquid-liquid phase split. The critical curve shows a temperature minimum and at temperatures above that temperature minimum two separate two-phase fluid-fluid regions are found. The low-pressure two-phase region shows an upper critical solution pressure, the high-pressure two-phase region shows a lower critical solution pressure. 2) To determine the applicability of HBPs as process solvents for CO 2 removal from gas streams, the vapor-liquid equilibria of different HBP – Co-solvent – CO 2 (Polyglycerol-Methanol-CO 2 ) systems are determined. Methanol is used as a co-solvent, in order to keep the viscosity sufficiently low. To describe this system, an extension of the PC-SAFT equation of state (EOS) is developed accounting for the branching within chain molecules. The applicability of the resulting EOS to describe the experimental data, determined as part of this study, is shown. Furthermore, the ability to extrapolate towards different solute and solvent concentrations as well as towards different molecular masses of the branched polymer is demonstrated. 3) Molecular Dynamics (MD) simulations along with Widom's test particle insertion method are used to compute the activity coefficient of CO 2 and N 2 at infinite dilution for various types of hyperbranched polymers. The TraPPE force field was used. We show that the simulation results are in reasonable agreement with experiments.
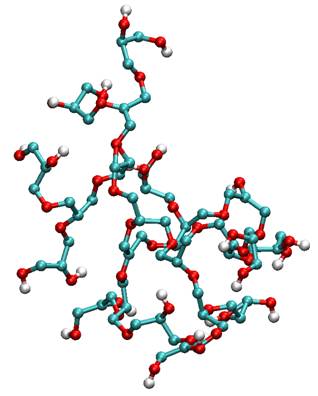
Figure 1. Typical MD snapshot of a single hyperbranched polymer. * corresponding author e-mail: t.w.deloos@tudelft.nl References Seiler et al., Separation and Purification Technology 30 , 179-197 (2003)
|
Envío de trabajos en línea
|
|
| English Version | Para mayor información: Luis A. Galicia-Luna (Chairman) |
© Escuela Superior de Ingeniería Química e Industrias Extractivas
Unidad Profesional Adolfo López Mateos Edif. 6, 7 y 8 Zacatenco C.P 07738 México, D.F.
Tel. 5729 6000 ext. 55294
| Resolución de pantalla recomendada: 1024x768 pixels |
Web Design by Nayeli JG |